19.02.2021
4 minutes of reading
Teams at IFPEN studied the phenomenon of fuel autoxidation, phase separation and deposit formation causing a variety of malfunctions. Having proposed a new deposit formation process, researchers validated it for diesel, biodiesel and aviation kerosene fuels using a new methodology and demonstrated the role of charge carriers and polarity at the oxidized/non-oxidized product interface.
Autoxidation, a source of deposits and malfunctions
Autoxidation refers to the phenomenon whereby a fuel tends to break down as a result of the oxidation of its compounds at low temperature (generally T < 300°C). This form of fuel degradation may result in the formation of insoluble products and deposits of diverse origin and compositions in tanks, combustion systems and injection circuits.
These deposits can then lead to mechanical malfunctions affecting pumps and injectors, filter clogging or malfunctions relating to increased pressure losses through filters.
Consequently, fuel stability in the context of autoxidation is a major issue. It concerns the entire logistics chain from storage through to its transportation and use in different modes of land, air and naval transport.
How are these deposits formed?
IFPEN has built up solid expertise in the field of fuel stability by studying deposit formation mechanisms. The objective: to gain a better understanding of the liquid phase autoxidation process and develop detailed, semi-detailed and global kinetic models designed to predict both fuel degradation as a function of its chemical composition and operating conditions as well as deposit formation.
Research conducted in recent years at IFPEN has mobilized a broad range of internal expertise1 and made it possible to demonstrate that deposits are primarily formed during the engine and turbine stop phases[2].
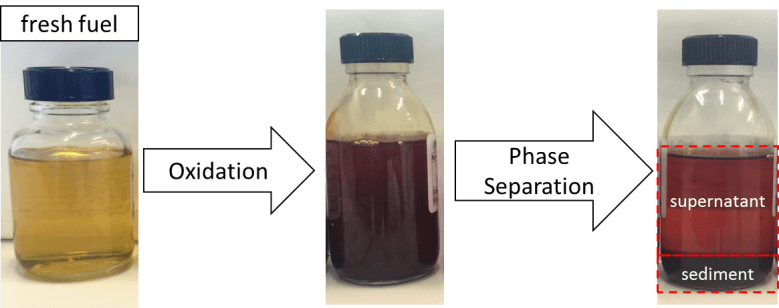
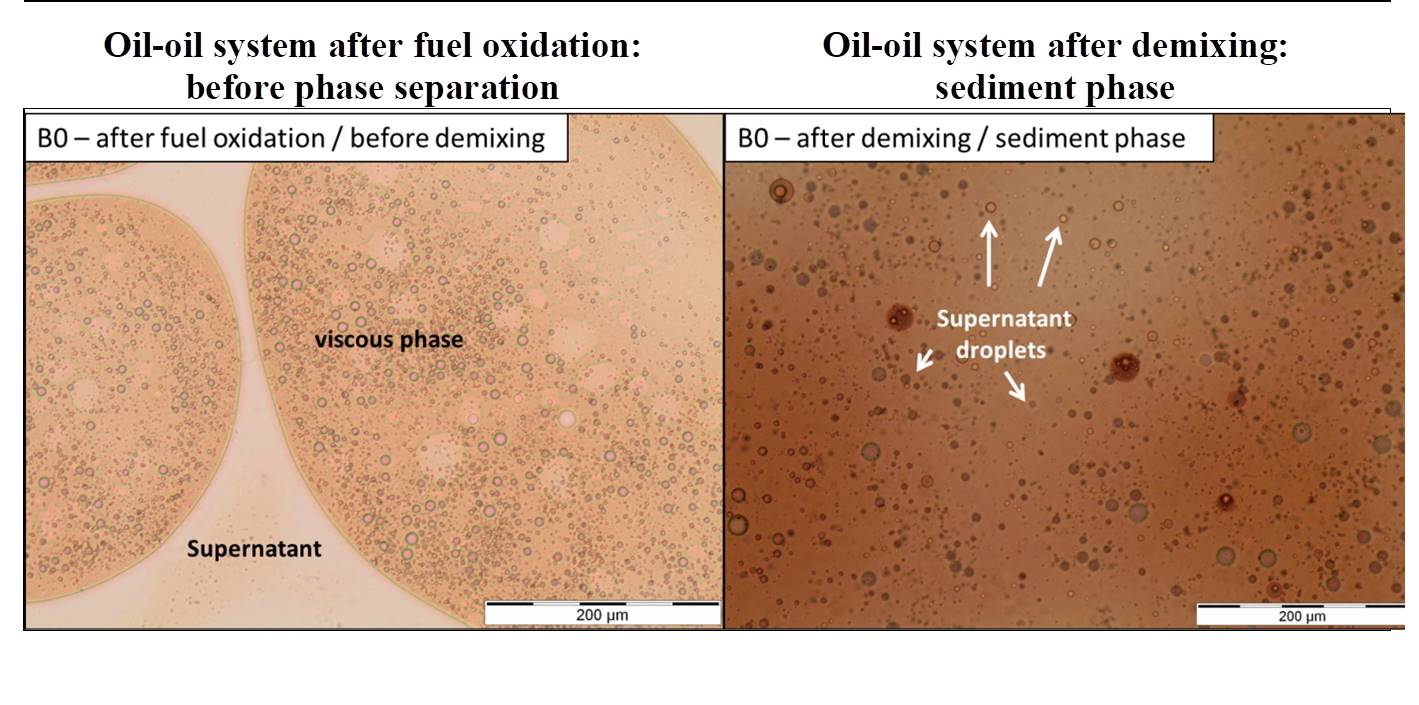
Accelerated oxidation methods employed in the laboratory2 involving real and model (surrogates) fuels made it possible to observe a liquid-liquid separation phase as a function of fuel oxidation conditions (figure 1). Detailed analyses of both the supernatant fractions and sedimented phases, particularly via GC-MS3, led to the identification of the broad chemical families of stable products formed during autoxidation processes: ketones, epoxides, aldehydes, alcohols and carboxylic acids[3–5]. These products appear to play an important role in the formation of several deposit precursors.
The characteristics of the aggregates do not, in themselves, explain phase separation
Research teams have exploited the complementary nature of molecular characterization techniques, such as GC-MS and FTIR4, and other approaches, such as low field NMR and X-ray scattering in order to improve our understanding of such mechanisms [6].
By way of example, small-angle scattering techniques, SAXS5 and SANS6 , made it possible to characterize the aggregation mechanisms demonstrated - diffusion limited aggregation (DLA) and reaction limited aggregation (RLA)[5] - as well as to monitor aggregate size, which increases in a linear manner with the number of particles in test conditions. Nevertheless, the physical characteristics alone (particle size and number) are not thought to explain the observed phase separation and deposits.
Polarity differences between oxidation products and the non-oxidized fluid
In order to identify the principal levers leading to phase demixing, IFPEN’s teams used optical microscopy as well as Turbiscan methods and FTIR[1]. Figure 2 shows the evolution via demixing of oxidized samples of a complex emulsion. Turbiscan results revealed that the demixing rate was not directly linked to aggregate density or viscosity. Instead, the phenomenon seemed to be primarily related to differences in polarity (due for example to oxygenated compounds) of the sedimentary and supernatant phase content, which was confirmed by the FTIR results.
Everything seems to indicate that deposit precursors are formed in the liquid phase, and, therefore, that the deposit is not directly generated by the surface in contact with the fuel. These observations led to the proposal of a new deposit formation mechanism [1]whereby the difference in polarity between the oxidation products and the non-oxidized fluid represents a significant phase separation lever (figure 3).
![Schéma du mécanisme proposé pour la formation des dépôts issus de la dégradation des carburants [1]](/sites/ifpen.fr/files/inline-images/Recherche%20fondamentale/StabiliteCarburant_AlvesFortunato3.png)
IFPEN validates a new deposit formation mechanism
To validate this mechanism proposal, experimental research was conducted using electrochemical impedance spectroscopy, combined with an electrochemical quartz crystal microbalance (EIS/EQCM). These combined techniques make it possible to analyze the dynamic behavior of a system. The idea is to apply an electrical disturbance to the system over time and monitor its response, as well as the mass of the deposit formed. This methodology highlighted the role of charge carriers and polarity at the oxidized/non-oxidized product interface on the deposit formation process for diesel, biodiesel and aviation kerosene fuels. Moreover, due to its efficacy, this methodological application has resulted in the filing of a patent[7].
In a part of the study dedicated to aviation fuels, EIS/EQCM coupling highlighted different processes leading to deposit formation on the microbalance from sulfur and aromatic compounds present in Jet A1. Capacitance (i.e., the polarity at the oxidized/non-oxidized product interface) seems to be the main parameter determining deposit formation.
This research has helped establish IFPEN as one of the leading players in the field of fuel stability in France and internationally.
1 Chemical analysis, structural analysis and imaging, complex fluids, colloids and condensed matter, surface, interface and materials science
2 PetroOxy or RSSOT, Rancimat, and Parr Autoclave
3 Gas chromatography (GC)-Mass spectrometry (MS) coupling
4 Fourier-Transform Infrared Spectroscopy
5 Small-Angle X-Ray Scattering
6 Small Angle Neutron Scattering
References :
[1] Alves-Fortunato M., Ayoub E., Bacha K., Mouret A., Dalmazzone C. Fatty Acids Methyl Esters (FAME) autoxidation : New insights on insoluble deposit formation process in biofuels, Fuel, 2020, 268, 117074. DOI: https://www.sciencedirect.com/science/article/abs/pii/S0016236120300697.
[2] Alves Fortunato M., Lenglet F., Ben Amara A., Starck L. Are Internal Diesel Injector Deposits (IDID) Mainly Linked to Biofuel Chemical Composition or/and Engine Operation Condition? International Powertrains, Fuels & Lubricants Meeting, JAN. 22, 2019. SAE International400 Commonwealth Drive, Warrendale, PA, United States, 2019.
[3] Karl CHATELAIN. Etude de la stabilité à l’oxydation des carburants en phase liquide. Paris: ENSTA ParisTech, 15/12/2016, 134 p.
[4] Kenza Bacha. Etude de l’Interaction entre le Carburant Diesel et les Composants du Système d’Injection Diesel. Université de Haute Alsace, 2016, 191 p.
[5] Alves-Fortunato M., Labaume J., Cologon P., Barré L. Biofuel Surrogate Oxidation : Insoluble Deposits Formation Studied by Small-Angle X-ray Scattering and Small Angle Neutron Scattering, Energy & Fuels, 2018. DOI: https://pubs.acs.org/doi/abs/10.1021/acs.energyfuels.5b02470.
[6] C. POURTIER, P. COLOGON, T. CHEVALIER, M. MATRAT, L. BARRE, Gaining a Detailed Understanding of Fuel Deposit Formation Mechanisms Through Advanced Molecular and Supramolecular Characterizations, 16th International Conference on Stability, Handling and use of Liquid Fuels (IASH), Long Beach, CA, 2019
[7] Alves Fortunato M., Ben Amara A., Mingant R., Sauvant-Moynot V., Starck L., Belaid S. PROCEDE ET SYSTEME POUR LA MESURE DE LA STABILITE A L'OXYDATION ET/OU DE LA STABILITE THERMIQUE D'UN CARBURANT. France, Brevet WO 2020/207813 A1, 15 octobre 2020.
Scientific Contact : Maira Alves Fortunato