Driven by the global challenge of switching to a more sustainable economic and energy model, IFPEN has been studying for a number of years biosourced products with high added value and working to develop processes for biomass recovery, as an alternative to conventional petrochemistry.
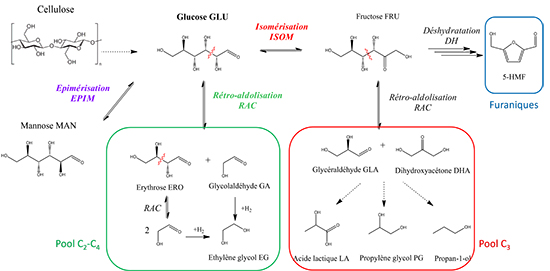
Sugars are one of the main components of lignocellulosic biomass, a key resource due to its non-competition with food production. In processes, they can react in different ways in order to obtain either motor fuels, or high added-value products such as furfural, ethylene glycol, propylene glycol or glycerol. Consequently, a major challenge in the conversion of these sugars lies in achieving optimal selectivity towards the desired products, hence the need of understanding the reaction mechanisms and the active species. In particular, the key factors at the molecular level that explain the selectivity of a specific catalyst towards a given reaction remain poorly understood.
As a monomer of cellulose and hemicellulose, glucose is the most abundant sugar of lignocellulosic biomass. It is the precursor of many different molecules with strong potential, through various catalytic conversions (figure 1). A doctoral thesis currently under way at IFPEN aims at gaining an understanding of and explain this particular selectivity of tungsten-based (W) catalysts in the conversion of glucose [1]. In fact, these catalysts effectively promote the reaction of retro-aldolisation, a key step in the production of ethylene glycol or short-chain synthons from sugars.
Through a combination of experimental techniquesa and molecular modelling by DFTb, we have been able to demonstrate the formation of specific complexes between sugars and metallic species, while also establishing the links with the various possible reaction pathways. In particular, the presence of multiple dinuclear complexes of tungsten has been shown (figure 2) and seems to be responsible for an epimerisation reaction, different to the retro-aldolisation reaction expected. In addition to this, it was also possible to propose transient mononuclear species for this retro-aldolisation reaction. X-ray absorption spectroscopy studies, as well as catalytic tests performed in various atmospheres, have also made it possible to specify that an oxydation degree of +VI is needed in tungsten species in order to effectively catalyze the reaction.
These mechanistic studies have motivated another project, which aims at studying the selectivity of the same reactions in the conversion of sugars, catalysed by zeolites displaying Lewis acidic sitesc. This project will be carried out from 2022 to 2025 as part of ANR’s JCJC programmed, and will also combine theory and experimentation work.
Click on figures to enlarge
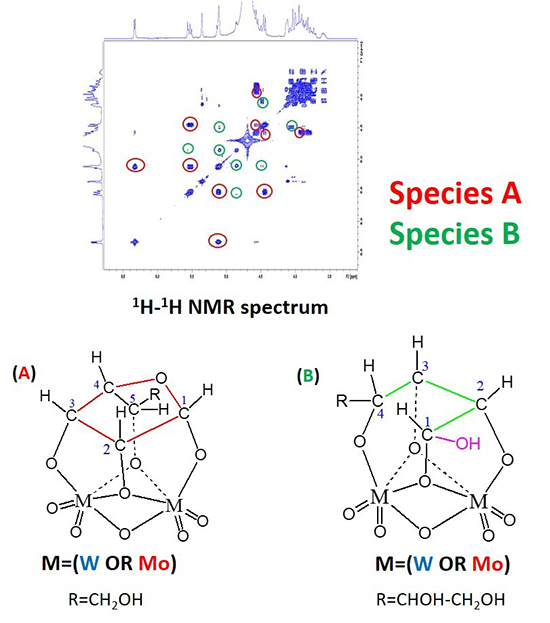
a- Including NMR spectroscopy in liquid state, kinetic measurements of the catalytic conversion of glucose and the in situ X-ray absorption spectroscopy
b- Density Functional Theory
c- Electron-accepting sites, allowing the formation of a covalent bond with electron donnor species (Lewis bases)
d- Young researchers
Reference:
- S. El Mohammad, K. Larmier, C. Chizallet, “Reactivity of glucose over tungsten- and molybdenum-based catalysts”, 5th European conference on Green and Sustainable Chemistry (EuGSC), 26 to 29 September 2021.
Scientific contacts: Maria Fernandez-Espada-Pastor, Kim Larmier
You may also be interested in
Hybrid catalysis can better deal with bio-based substances
THÈSE DE MARIE GUEHL







