Thesis prepared by Julien Petit:"Exploration of a new ethylene oligomerization reactivity – Towards dicationic nickel complexes"
The olefin oligomerization reaction provides access to a broad range of key compounds in the fuel, petrochemical and fine chemistry sectors.
In these different contexts, the rich and unique reactivity of nickel with unsaturated compounds has made this metal one of the principal success stories for this family of catalytic processes. On an industrial scale, the selective oligomerization of ethylene to 1-butene, 1-hexene or 1-octene (intermediates in the manufacture of different grades of polyethylene) respectively currently uses metals such as Ti and Cr. In this field, Ni-based catalysts are not yet used since they are too rarely selective to a single olefin and sometimes highly isomerizing, which reduces the quality of the α-olefins produced (required purity > 99.3%).
PhD research conducted at LHFAa (University of Toulouse) and IFPEN was aimed at studying the possibility of having these nickel catalysts function according to a mechanism known as “oxidative coupling”, recognized as being more selective but yet to be demonstrated for this metal [1]. On the basis of preliminary theoretical calculations (using DFTb ), it was possible to identify the [LnNi2+] fragment (dicationic Ni(II) complex associated with n L-type ligands) as a potential intermediate for the oxidative coupling of two ethylene molecules, in order to obtain 1-butene selectively.
While dicationic nickel (II) species are reported in the literature, they are generally stabilized by at least four L-type strong electron donating ligands, preventing any ethylene molecule from coordinating at the metal center (Ni2+). This research thus set out to design access paths to [LnNi2+] species, with at least two coordination sites accessible for ethylene. Access to these Lewis acid compoundsc was explored using diverse approaches (Figure).
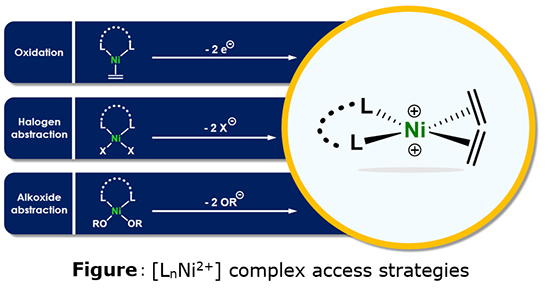
[LnNi2+] complexes, little described in the literature, were synthetized and totally characterized by a combination of techniques: NMR, mass spectrometry, X-ray diffraction as well as DFT studies. They were then employed in ethylene oligomerization reactions. Remarkably, some complexes were found to be active for butene production, paving the way for a new reaction path that could not yet be totally identified at this stage, however [2]. This highly exploratory research confronted nickel with what remains an extremely challenging fundamental objective today.
In 2021, Julien Petit was awarded the French Chemical Society’s Coordination Chemistry Division prize.
a- LHFA: Laboratoire Hétérochimie Fondamentale et Appliquée or Fundamental and Applied Heterochemistry Laboratory.
b- DFT: Density-functional theory.
c- Species likely to accept an electron pair.
Bibliographic references:
-
J. Petit, L. Magna, N. Mézailles, "Alkene oligomerization via metallacycles: Recent advances and mechanistic insights" Coor. Chem. Rev., 450, (2022), 214227
>> https://doi.org/10.1016/j.ccr.2021.214227
-
J. Petit, N. Saffon-Merceron, L. Magna, N. Mézailles "Synthesis of L2Ni(ORF)2 (RF = C(CF3)3) Complexes and Their Reactivity in Ethylene Oligomerization" Organometallics, 2021, 40, 4133−4142
>> https://doi.org/10.1021/acs.organomet.1c00575
Scientific contact: lionel.magna@ifpen.fr







