The dehydration of bio-based alcohols to form alkenes is a key reaction to obtain major chemical intermediates from biomass. It is efficiently catalyzed by zeolites presenting Brønsted1 acid sites and a crucial challenge is the control of its selectivity. Starting, for example, with a branched alcohol like isobutanol, the dehydration reaction does not only produce isobutene (branched isomer from the butene family), as expected by conservation of the molecule’s carbon skeleton (Figure 1 (a)). Therefore, despite operating conditions in which isobutene should not be isomerized into linear alkenes, this reaction also produces linear butenes.
Linear alkenes are highly sought-after intermediates in the chemicals industry. As a result, it is important to identify the mechanisms making it possible to produce them via the conversion of isobutanol since this knowledge is required to control the selectivity of these reactions.
Conducted in collaboration with Comenius University of Bratislava (team led by T. Bučko), research focusing on the mechanisms behind the conversion of isobutanol to alkenes used density functional theory (DFT), based on a model zeolite, of the chabazite type. As a result, important transition states were identified, as well as corresponding free energy barriers [1]
This approach made it possible to identify two major families of new mechanisms capable of explaining the formation of linear alkenes from isobutanol:
- a sequential mechanism of isomerization of isobutanol into other butanols, particularly 2-butanol (Figure 1 (b)), a linear alcohol that can then be dehydrated into linear alkenes,
- a synchronous isobutanol isomerization and dehydration mechanism making it possible to form linear alkenes directly.
The analysis of free energy profiles made it possible to find the most favorable paths. In typical dehydration conditions, the DFT calculation suggests that the first mechanism represents the fastest reaction route.
These results explain the reaction products observed and pave the way for selectivity control for linear alkene production.
The precise quantification of rate constants is now necessary in order to predict the activity and selectivity of such a catalyst, via a kinetic model. This quantification requires an investigation of these new mechanisms through ab initio molecular dynamics, in line with previous research relating to alkene isomerization and cracking.
In addition, the influence of the nature of the zeolite still requires analysis. That is because while the simulation was based on chabazite, it is known experimentally that ferrierite zeolite presents a high degree of linear alkene selectivity. The role of the external surface of zeolite crystallites is thus questioned [2, 3] and research is being conducted within the context of the ongoing collaboration with Bratislava University. This role is being studied via the modeling of the reactivity of the external ferrierite surfaces. A visiting PhD student from Bratislava University spent the last quarter of 2022 at IFPEN focusing on this research.
This study will also be continued within the framework of the MAMABIO project (IFPEN, Ecole des Ponts ParisTech, Lorraine University, Caen University collaboration) within the B-BEST Priority Research Program and Infrastructure (PEPR).
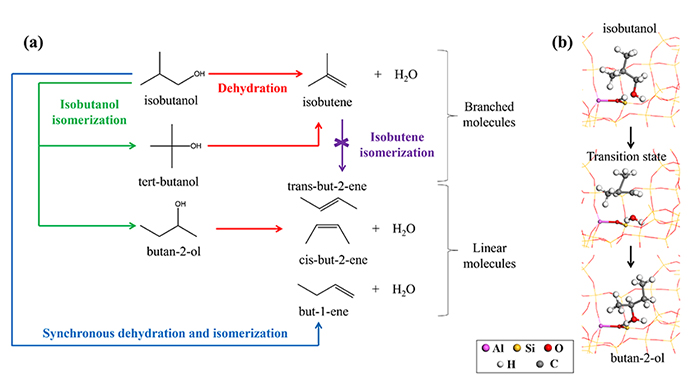
(b) structures calculated with DFT concerning the isobutanol isomerization to 2-butanol reaction.
1- “Proton donor”-type acid
References:
-
M. Gešvandtnerová, T. Bučko, P. Raybaud, C. Chizallet, Monomolecular mechanisms of isobutanol conversion to butenes catalyzed by acidic zeolites: Alcohol isomerization as a key to the production of linear butenes, J. Catal., 413, 786, 2022.
>> https://doi.org/10.1016/j.jcat.2022.07.025
-
S. Van Daele, D. Minoux, N. Nesterenko, S. Maury, V. Coupard, V. Valtchev, A. Travert, J.-P. Gilson, A highly selective FER-based catalyst to produce n-butenes from isobutanol, Appl. Catal. B, 284, 119699, 2021.
>> https://doi.org/10.1016/j.apcatb.2020.119699
-
Z. Buniazet, A. Cabiac, S. Maury, D. Bianchi, S. Loridant, Unexpected selectivity of ferrierite for the conversion of isobutanol to linear butenes and water effects, Appl. Catal. B, 243, 594, 2019.
>> https://doi.org/10.1016/j.apcatb.2018.11.007
Scientific Contact: Céline Chizallet
You may also be interested in
Predicting performance from atomic through to reactor scales to improve industrial processes
Shedding light on the external surface of zeolites by quantum calculation
Molecular modelling: a key tool for current and future heterogeneous catalysis
Chemical theory at quantum level (density functional theory or DFT) is an essential tool in rationalising the reaction mechanisms involved in the preparation of catalysts, as well as in their use, thanks to the optimisation of their activity [1,2]. IFPEN has carried out a number of projects aiming to shed light on these catalysts, which are of particular interest to industrial processes...