26.07.2023
3 minutes of reading
Researchers from IFPEN's Catalysis, Biocatalysis and Separation division have recently published a state-of-the-art report on transformations catalyzed by Brønsted acids in zeolites. Published in the journal Chemical Reviews, this body of knowledge illustrates IFPEN's growing involvement in the energy and ecological transition.
State-of-the-art article published
Published in the special issue Bridging the Gaps: Learning from Catalysis across Boundaries of the journal Chemical Reviews, the article presents an overview of the current state of knowledge on the mechanisms of reactions catalyzed by Brønsted acids in zeolites. This subject has been the focus of major research efforts by the scientific community for decades, and has many potential applications.
As well as being of practical interest to those working in the field of chemical and process innovation, this publication illustrates IFPEN's involvement in research challenges that are vital to transforming the industry in response to climate change.
It also reflects the recognition of IFPEN teams by the international scientific community
Catalysis: a field of chemistry at the heart of the ecological and energy transition
At the heart of IFPEN's R&I, catalysis is regularly in the spotlight, with researchers receiving awards for their work. Catalysis offers a wide range of benefits for a more sustainable energy future. For example, it lowers the activation energies required to initiate chemical reactions, and improves their speed and selectivity (reducing the formation of other molecules/waste). It also facilitates the biobased production of fuels and chemical intermediates. In addition, it is involved in processes that convert CO2 into fuel.
An innovative molecular approach
The authors have adopted an innovative molecular approach, bringing together both experimental and computational methods. The elements reported thus emanate from several types of techniques: detailed kinetic studies, in situ and operando spectroscopies, quantum chemical calculations.
These include the identification of relevant intermediates and transition states, and the respective kinetics of their formation and transformation into products.
Detailed characterization of sites and phenomena at work
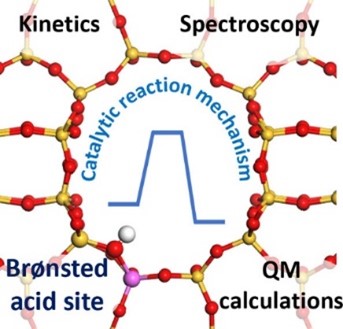
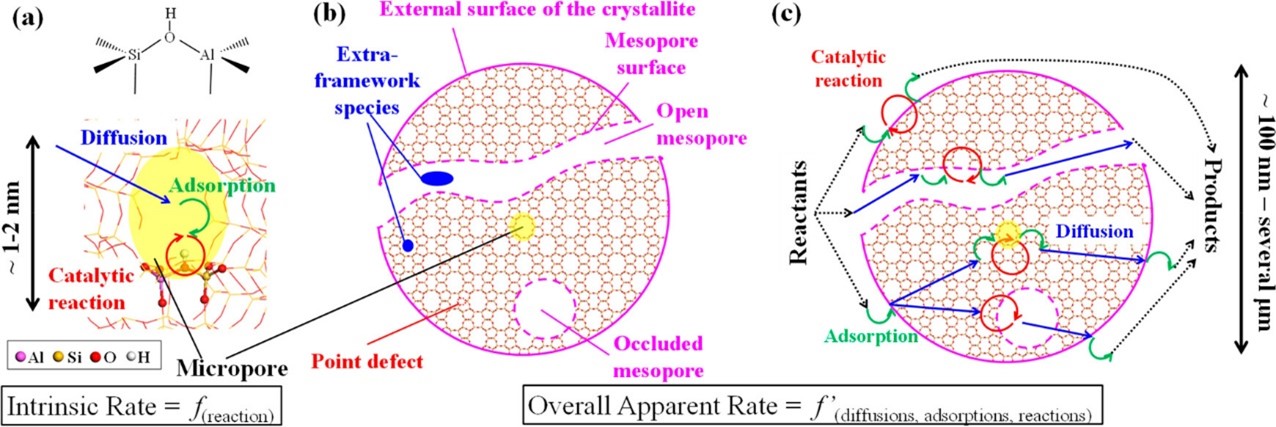
When it comes to zeolite catalysis, the structure, location and accessibility of active sites is a key consideration. This aspect has been extensively addressed, providing insights into the nature and acidity of possible active sites, as well as the role of the various phenomena at work: adsorption, diffusion, reaction kinetics.
The figure below outlines the most relevant concepts in this field, which are important for understanding the approaches undertaken, the knowledge obtained to date and the remaining challenges for understanding the mechanisms catalyzed by zeolites in their protonic form.
Important reactions for industrial applications
A large part of the paper is devoted to the description of atomic-scale mechanisms for reactions of great industrial importance, such as:
- conversion of alkenes, alkanes and aromatic molecules;
- dehydration reactions of alcohols and polyhydroxylated molecules;
- carbonylation reactions;
- reactions involved in the conversion of methanol to olefins.
Reference :
(1) Chizallet C., Bouchy C., Larmier K., Pirngruber G. (2023). Molecular Views on Mechanisms of Brønsted Acid-Catalyzed Reactions in Zeolites, Chem. Rev. 2023, 123, 9, 6107–6196 30, 2023, https://doi.org/10.1021/acs.chemrev.2c00896
Scientific contact : Céline Chizallet
You may also be interested in
Hybrid catalysis can better deal with bio-based substances
THÈSE DE MARIE GUEHL
More efficient catalysts: ifpen researchers elucidate the mechanism behind defect formation in zeolites
New acid zeolites obtained from silicogermanates
Zeolites are microporous crystalline aluminosilicates that exist in the natural state and can also be synthesised for a wide range of applications, from the biomedical industry to the production of renewable energy. (...) This work was carried out entirely at IFPEN as part of a doctoral thesis and has resulted in a new stable zeolite...